ASTM F732: Standard Test Method for Wear Testing of Polymeric Materials Used in Total Joint Prostheses
This test method describes a laboratory method for evaluating the wear properties of polymeric materials intended for use as bearing surfaces in human total joint prostheses. The standard utilizes simplified specimen geometries to rank material combinations based on their wear rates under simulated physiological conditions.
Significance and Use
This test method serves as a crucial screening tool. It allows researchers and manufacturers to quickly and reliably evaluate candidate materials and surface finishes before committing to expensive and time-consuming testing in full joint simulators. While it simplifies contact geometries and motions, it is intended to rank polymer wear rates effectively within specific wear applications.
Key Test Applications
The standard includes specific annexes for different wear applications:
Delamination Wear (Annex A3): Assesses a material’s resistance to delamination (cracking and removal of surface sheets), a mechanism often observed in oxidized polymers or incongruent contacts.
Linear Reciprocating Motion (Annex A1): Evaluates matierla for devices experiencing straight or rotatory reciprocating motion, such as hinged knees or trunnion bearings.
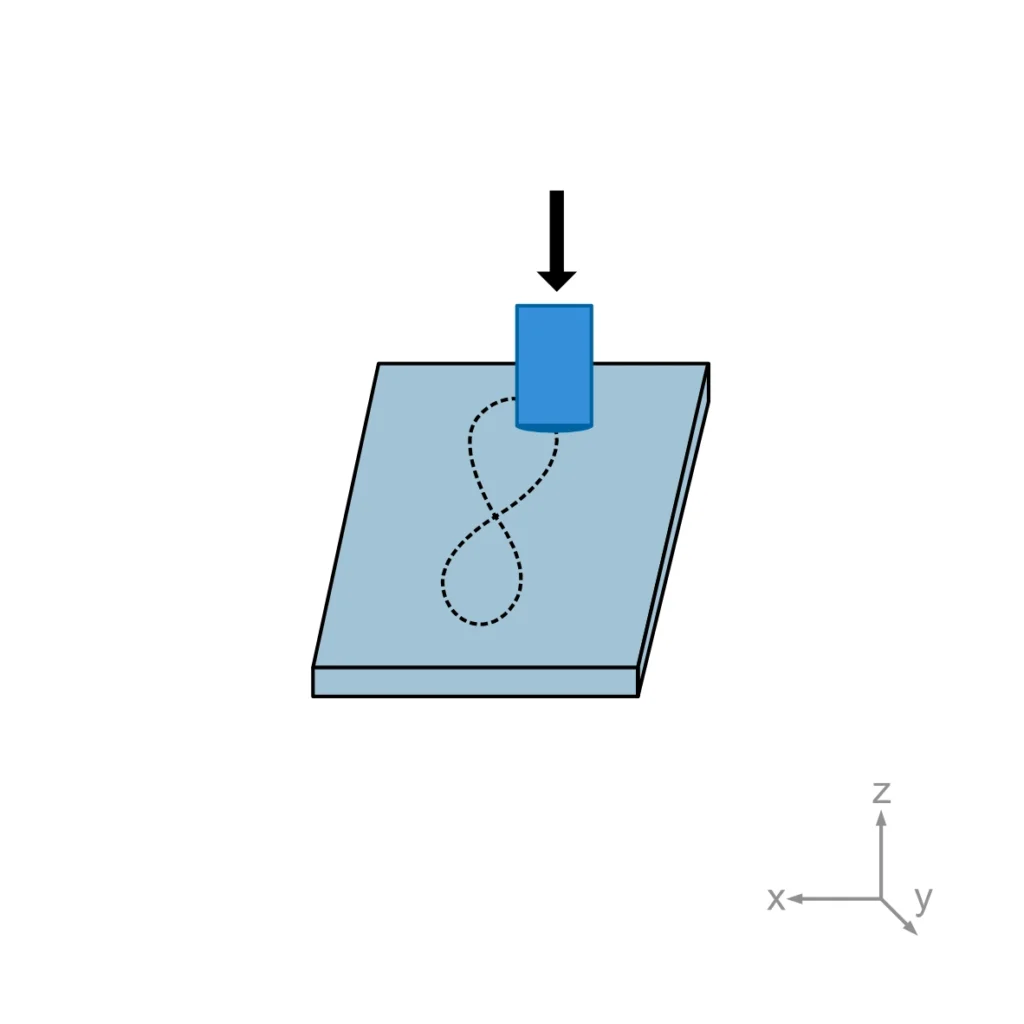
Fixed-Bearing Ball-Cup (Annex A2): Focuses on “hip-type” applications requiring multidirectional motion and cross-shear to simualte wear in total hip replacements.
Methodology Highlights:
- Lubrication: Tests are typically conducted using bovine serum to replicate the lubricating properties of physiological fluids.
- Fluid Sorption Correction: Because polymers may absorb fluid, soak control specimens are used to correct weight measurements and ensure accuracy.
- Measurement: Wear is typically reported as gravimetric (weight) loss