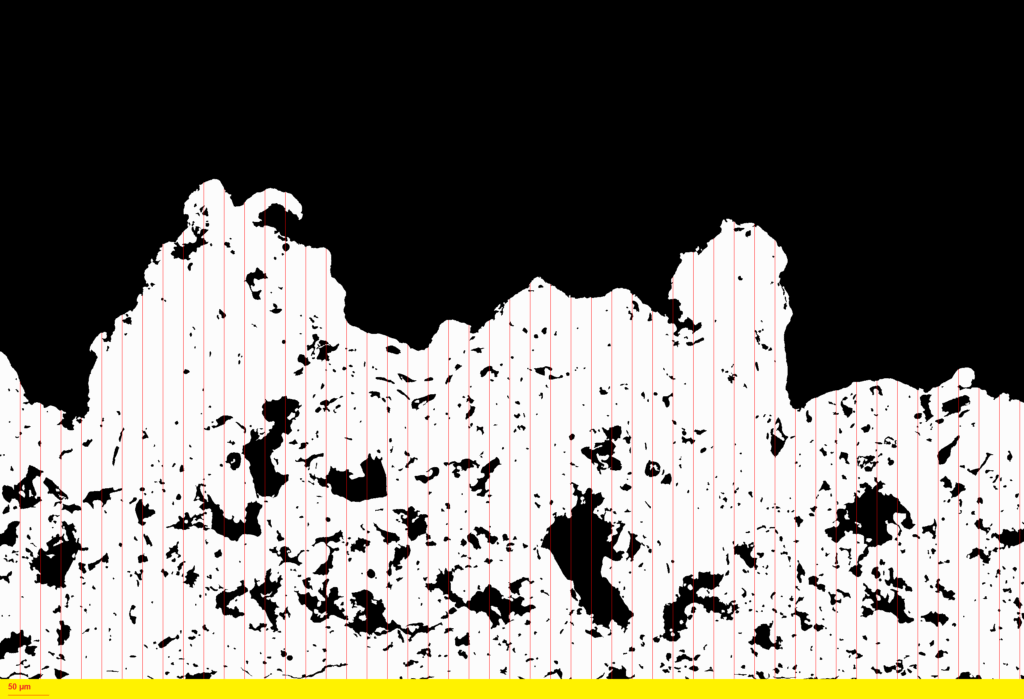
ASTM F1854: Standard Test Method for Stereological Evaluation of Porous Coatings on Medical Implants.
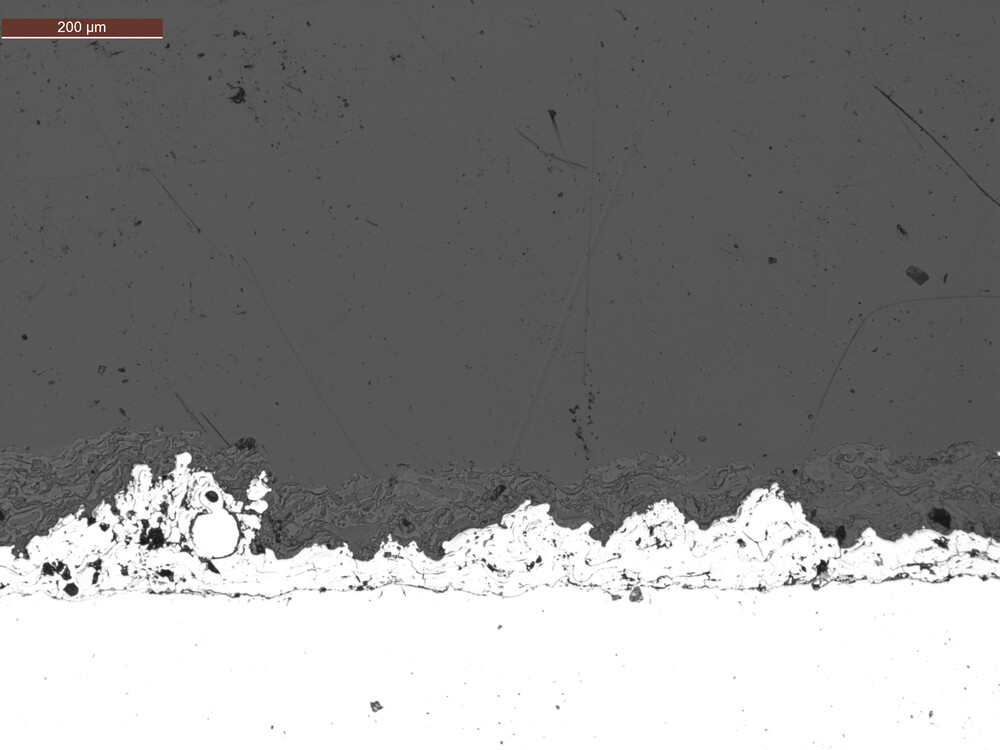
ASTM F1854 outlines standardized stereological methods for quantitatively assessing porous coatings applied to medical implants. The standard outlines procedures for determining key parameters, including coating thickness, volume percent void, and mean void intercept length. For coatings thicker than 500 µm, it also includes methods to evaluate the tissue interface gradient.
At EndoLab®, we offer tailored preparation methods specifically designed for typical implant coatings, including plasma-sprayed titanium, hydroxyapatite, and additively manufactured porous structures. Achieving accurate results begins with flawless metallographic preparation, where precision is paramount. To this end, we utilize dedicated high-resolution microscopes capable of resolutions better than 1 µm/pixel, enabling highly precise analysis.
To ensure both accuracy and repeatability, we do not rely on manual evaluation techniques. Instead, we have developed a proprietary image analysis software that automates the stereological evaluation process in compliance with ASTM F1854. This approach minimizes variability and ensures robust, reproducible results for critical implant surface assessments.