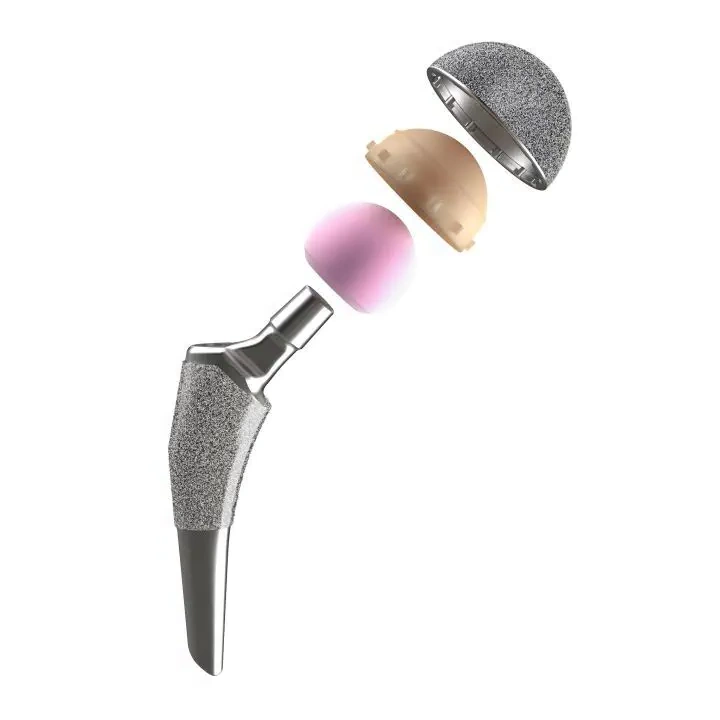
ISO 21535: Non-active surgical implants – Joint replacement implants – Specific requirements for hip-joint implants
ISO 21535 has been fundamentally revised. It now serves as the go-to standard for all hip devices, and includes the relevant ASTM procedures. For submission to regulatory bodies worldwide, ISO 21535 (the so-called “hip umbrella standard”) is now the applicable guideline.
Additionally, the standard outlines the evaluation process for test results, which involves a multi-stage procedure. In particular, it requires the use of a reference implant (predicate device), which presents manufacturers with fundamentally new requirements.
Our specialists will be happy to assist you in selecting the worst-case combinations for testing and to be tested and in developing a test plan that meets the requirements of ISO 21535.