ISO 22622: Implants for surgery – Wear of total ankle-joint prostheses – Loading and displacement parameters for wear-testing machines with load or displacement control and corresponding environmental conditions for test
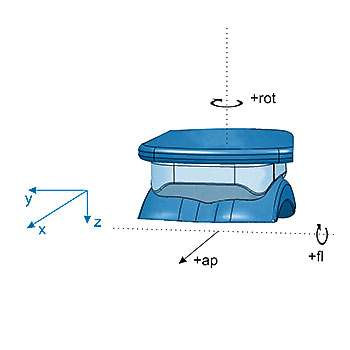
The wear of total ankle joint replacements under standard gait conditions can be determined using the EndoLab® knee joint simulator. Simultaneous testing of three (plus soak control) specimens enables rapid development, saving money and time. Routine testing is performed using bovine serum as a test fluid. In general, the test is stopped after 5 million cycles. Wear is determined by weight loss measurements. Standard inspection cycles are 0.5 million cycles. Optionally, particle analysis can be performed.