ASTM F543: Standard Specification and Test Methods for Metallic Medical Bone Screws.
This specification provides requirements for materials, finish and marking, care and handling, and the acceptable dimensions and tolerances for metallic bone screws that are implanted into bone. The dimensions and tolerances in this specification are applicable only to metallic bone screws described in this specification.
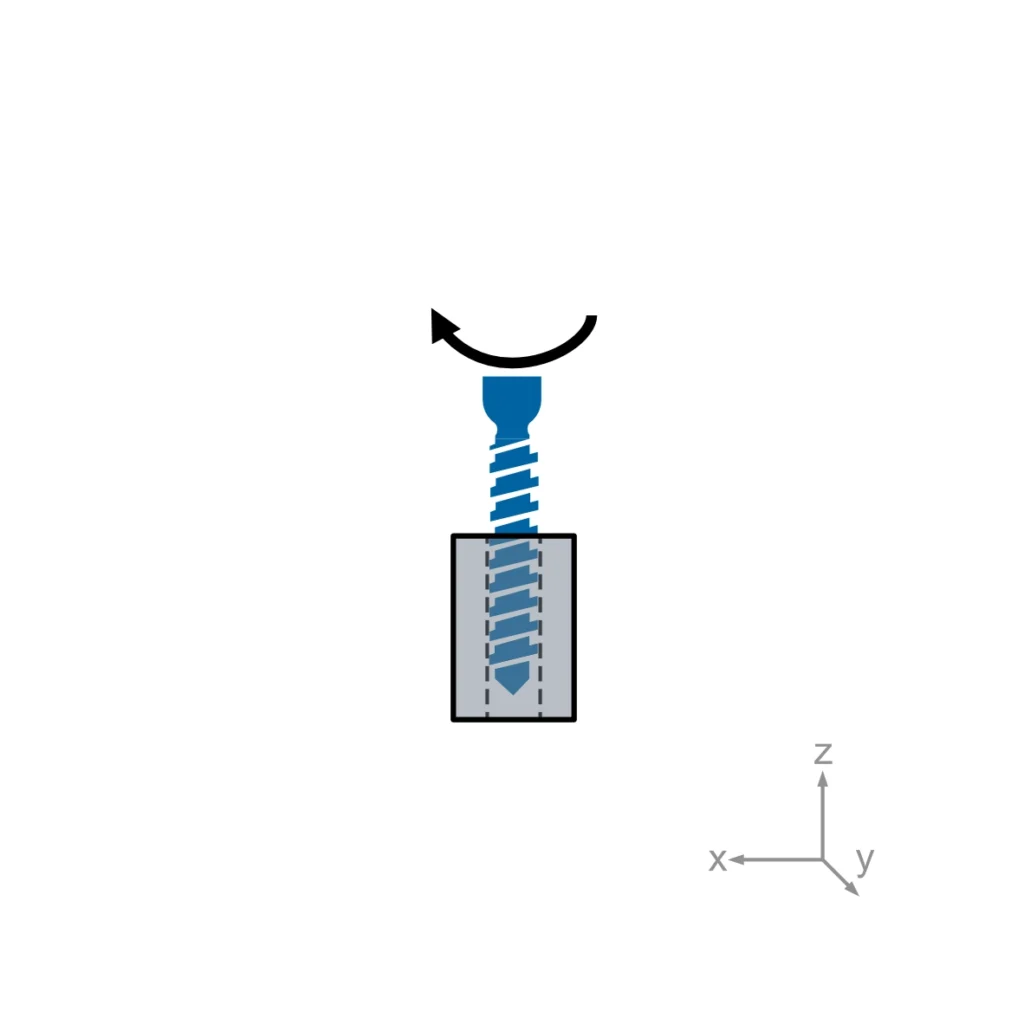
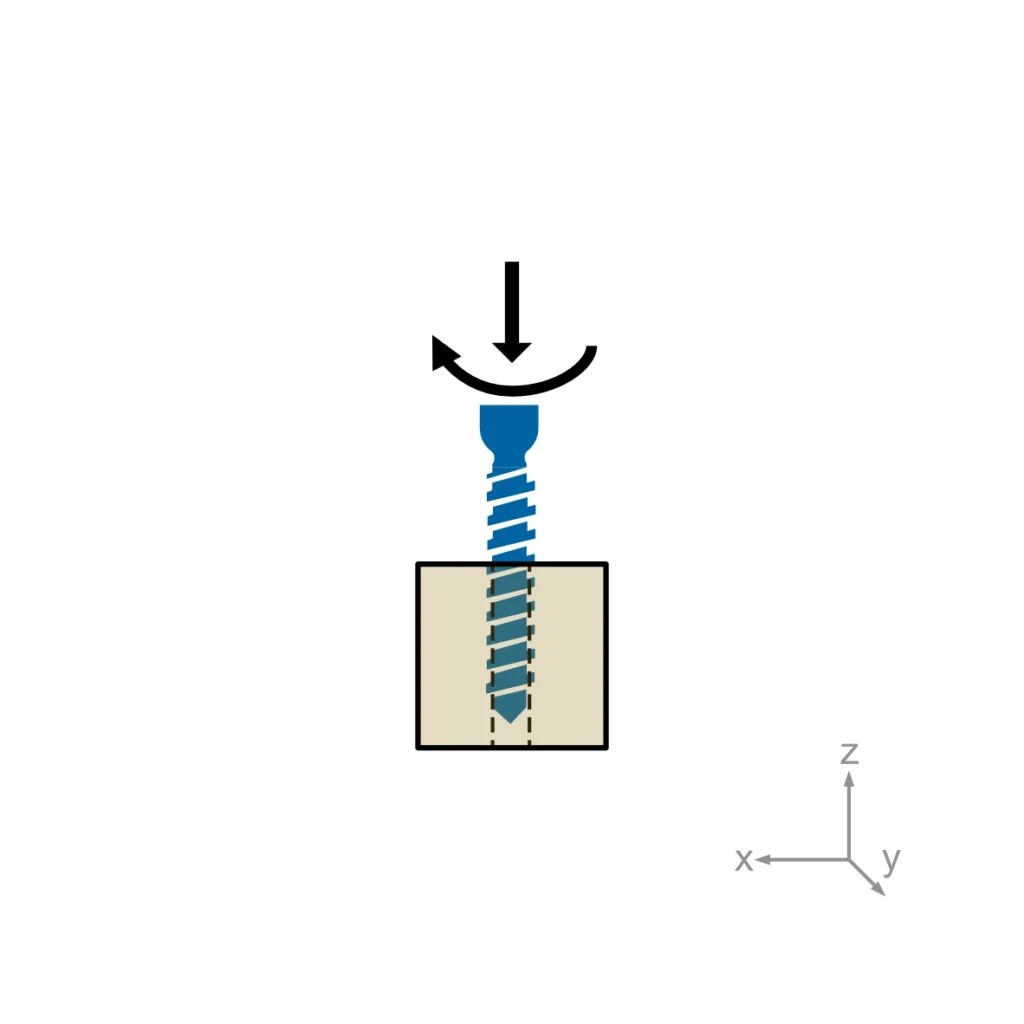
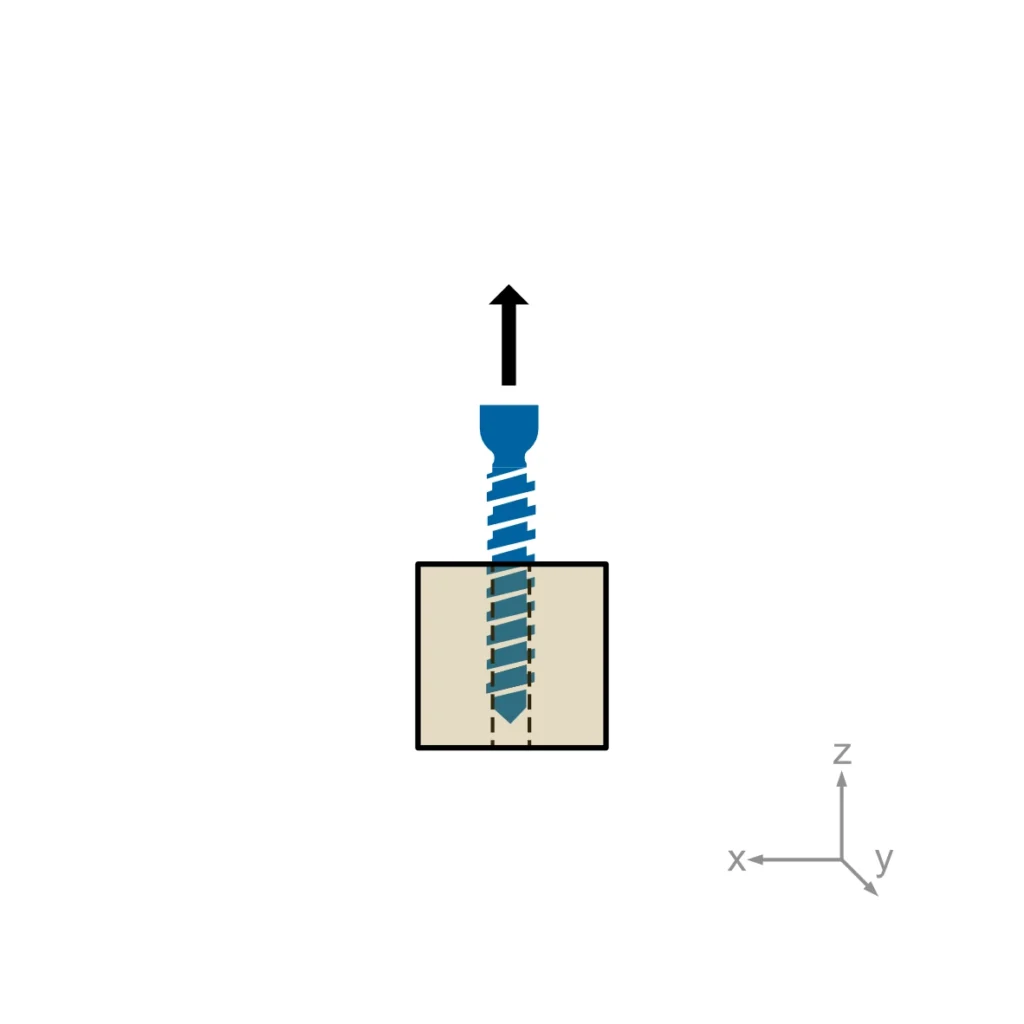
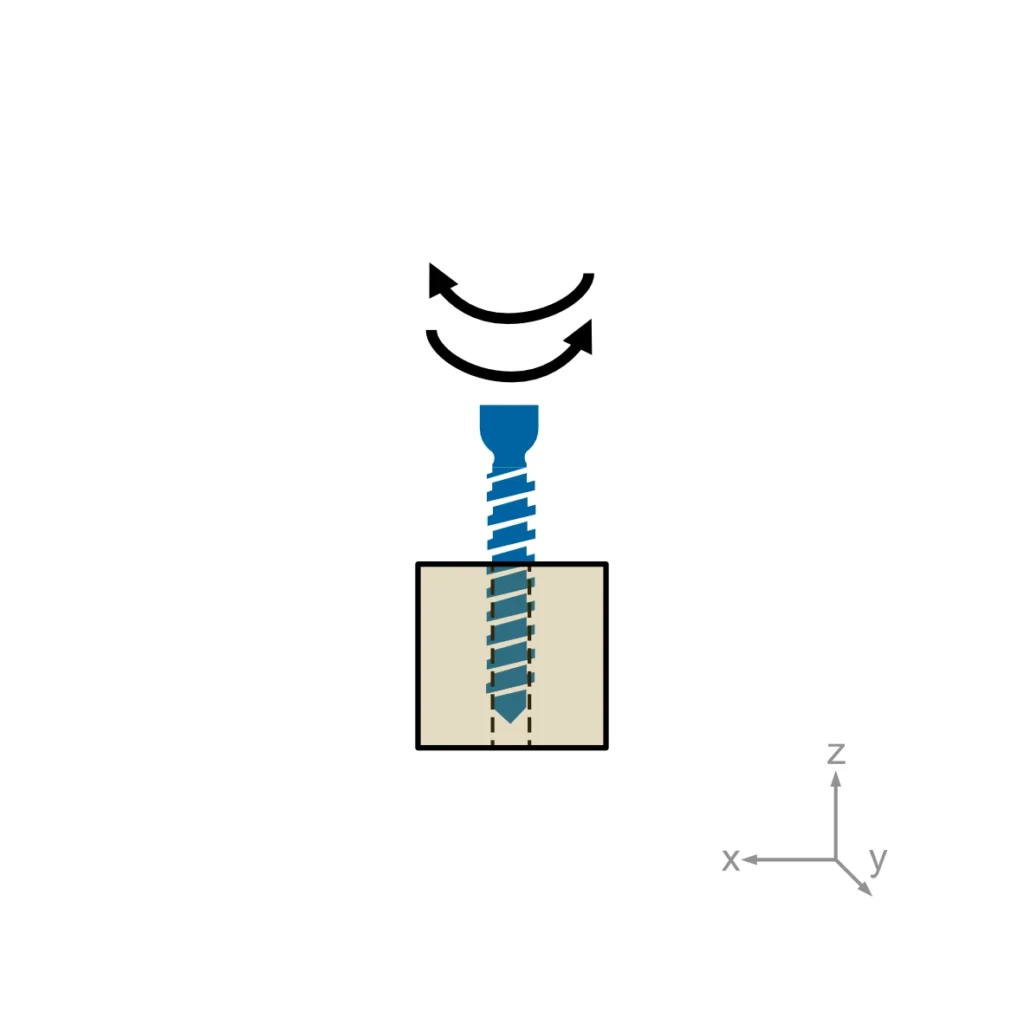
ASTM F543 describes four different test methods for bone screws: The determination of the torsional properties, driving torque and axial pull-out strength of medical bone screws. A self-tapping performance test needs to be performed for self-tapping medical bone screws only.
Reference data can be found in the EndoLab® database as well as in the literature.
Usually, torsional properties, driving torque, and axial pullout load are required for regulatory submissions. Self-tapping performance is added when relevant.
Driving torque: ≤ 50% of torsional yield strength
Pullout & torsion: Must meet FDA criteria or match predicate devices
Diameter, thread shape, and flute sharpness all affect performance. Even small differences can change insertion depth or torque. We have compiled more information on this in the whitepaper below.
Titanium and stainless steel are common for bone screws. Titanium generally shows higher torsional yield strength in tested samples. More information on the material differences can be found in our free pdf whitepaper.
Yes and No.
The FDA allows analytical pullout strength calculations. For this, you are welcome to use our template, which you can download below.
However, physical testing is recommended and often required by other agencies. Physical testing also provides a wealth of other benefits, since it not only tests the product but ultimately, the entire manufacturing process. In our experience, analytical predictions frequently do not match experimental values.
For a comprehensive summary, we have published a whitepaper, which you can find below.
Minor differences, such as dull flutes, can significantly alter torque and insertion behavior. To learn more about this, download our whitepaper below.
Test screws must be sterilized and match final production methods in geometry, surface finish, and tooling.
Yes, this standard applies to bone screws in general, including special cases such as screw anchors or marker screws.
Other standards apply to pedicle screws, orthodontic anchor screws, and absorbable screws.
Absolutely! For example, driving torque and pull-out results can be used to optimize the thread geometry.
Yes! There is an FDA Guidance for Non-Spinal Metallic Bone Screws and Washers at https://www.fda.gov/media/130866/download. Additionally, you can download our free pdf whitepaper below.