ASTM F2996: Standard Practice for Finite Element Analysis (FEA) of Non-Modular Metallic Orthopaedic Hip Femoral Stems
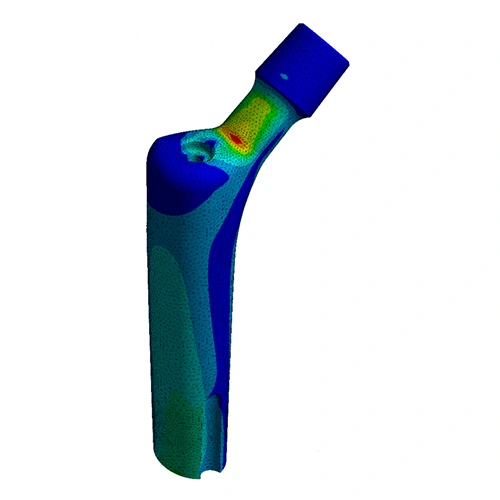
This standard establishes requirements and considerations for developing Finite Element models to evaluate static implant stresses and strains of non-modular metallic orthopaedic hip stem designs. It can be used for worst-case assessment within a family of implant sizes to reduce the need for physical testing. The boundary conditions are set-up according to ISO 7206-4.
The verification and validation of computational models is an essential component for an accurate simulation. To establish model credibility, EndoLab follows the guidelines described in ASME V&V40.
ASTM F2996 is used to determine the worst-case implant combination for testing hip femoral stem fatigue according to ISO 7206-4.
ASTM F2996 is not mandatory for FDA clearance; however, the use of ASTM F2996 can provide quantitative evidence for the appropriate selection of the worst-case combination.
Dual taper designs are outside of the scope of ASTM F2996; however, EndoLab has the knowledge to simulate complex modular systems.