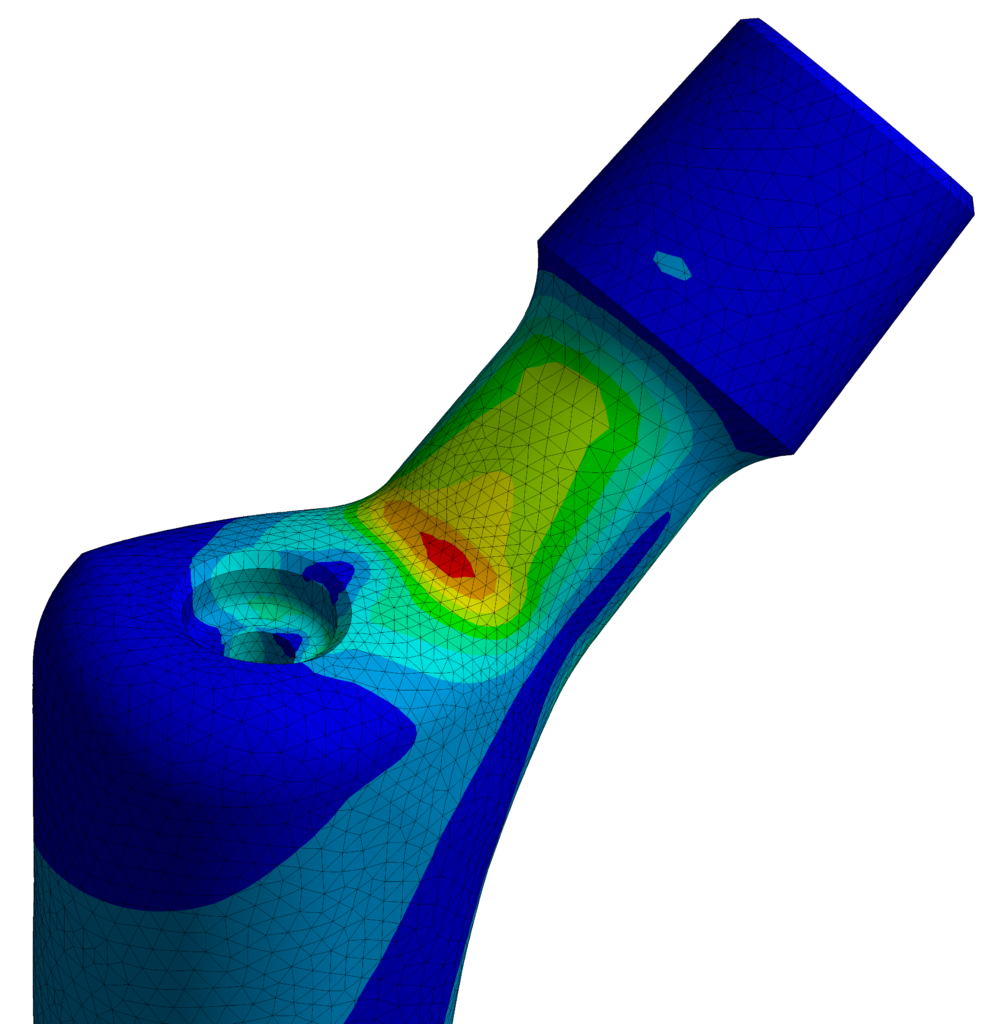
Analogous to ASTM F2996, which simulates the physical testing of ISO 7206-4, EndoLab has developed a protocol to evaluate the static implant stresses and strains of non-modular metallic orthopaedic hip stem designs in the femoral neck region. The boundary conditions are setup according to ISO 7206-6.
The verification and validation of computational models is an essential component for an accurate simulation. To establish model credibility, EndoLab follows the guidelines described in ASME V&V40.
PI-87 is EndoLab’s internal FEA (Finite Element Analysis) protocol for non-modular metallic hip stems. It is based on ASTM F2996 but specifically focuses on the femoral neck area, a region for which no standard FEA method currently exists. PI-87 can also help identify the worst-case scenarios to guide mechanical testing in according to ISO 7206-6.
No, PI-87 is not mandatory for regulatory submissions. It is a custom EndoLab protocol that can complement recognized standards (such as F2996) when preparing submissions.