Particle analysis
Normative References
ISO 17853: Wear of implant materials - Polymer and metal wear particles - Isolation, characterization and quantification.
ASTM F1877: Standard Practice for Characterization of Particles.
ASTM F561: Standard Practice for Retrieval and Analysis of Medical Devices, and Associated Tissues and Fluids
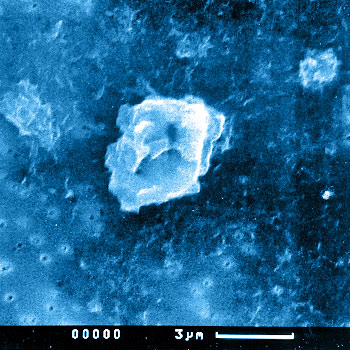
Besides the total wear volume determined by simulator tests, the number and morphology of the wear debris are major factors influencing the long term stability of joint replacements. After predefined numbers of cycles, the serum can be analyzed.
The morphology of the particles can be described using characteristic values like equivalent circle diameter (ECD), the aspect ratio (AR), the elongation (E), roundness (R) and form factor (FF).
Procedure for polymer materials:
The acid digestion method is used for the preparation of polymer particles. Using a 0.05µm filter, the particles can be separated from the fluid. Scanning electron microscopy (magnification 5,000x) is used to gain digitized images of the particles. Subsequent image analysis enables the determination of shape and distribution values.
Procedure for metal materials:
Metal particles are separated using an enzymatic process. Concentration and purification of the particles is achieved by centrifugation. Scanning electron microscopy is used for imaging the particles followed by image analysis to determine the size distribution. EDAX is used to distinguish between wear particles and artefacts.